BIOPROCESS ENGINEERING
Ancient Fermented Products
- 7000 BC: Sumerians/Babylonians were making beer, marking one of the earliest known uses of fermentation.
- 6000 BC: Wine production in Georgia.
- 4000 BC: Egyptians used yeast to leaven bread.
- 3000 BC: Babylonians produced fermented milk products.
- 1000 BC: Fermented soybean curd (precursor to tofu) was developed.
- 400 BC: Vinegar production began.
Medieval and Early Modern Advances
14th Century:
- Development of the Orléans process for vinegar fermentation.
- Alcohol distillation became prominent.
17th Century:
- Antonie van Leeuwenhoek invented the microscope, enabling the study of microbes.
19th Century Milestones
Schwann and Theodor (1840):
- Demonstrated that microbes are responsible for fermentation.
Justus von Liebig:
- Proposed the chemical hypothesis of fermentation.
Louis Pasteur (1857–1876):
- Proved yeast drives the fermentation process.
- Disproved the chemical hypothesis and the theory of spontaneous generation.
Hansen of Carlsberg Brewery:
- Isolated and propagated single yeast cells for consistent brewing.
20th Century Innovations
Early 1900s (Chaim Weizmann):
- Known as the "Father of Industrial Fermentation."
- Developed acetone and butanol fermentation using flax and hemp.
- Introduced the steel fermenter, leading to modern industrial fermentation.
1929 (Alexander Fleming):
- Discovered penicillin.
- W. Florey and B. Chain (1939) stabilized penicillin and demonstrated its safety in mice.
Strain Improvement:
- Enhanced microbial strains for higher penicillin yields:
- E.g., P. chrysogenum strains increased from 60 μg/ml (1951) to 1800 μg/ml (Wisconsin family strains).
- Enhanced microbial strains for higher penicillin yields:
SCP (Single-Cell Protein) Production (1940–1964):
- Development of massive fermenters with capacities up to 150,000 liters.
1964–1979:
- Focus on metabolite production:
- Amino acids, nucleosides, enzymes, and biopolymers (like dextran).
- Innovations like enzyme immobilization.
- Focus on metabolite production:
Biotechnology Era (1980s Onwards)
Genetic Engineering:
- Revolutionized industrial fermentation by enabling the creation of genetically modified organisms (GMOs).
rDNA Technology:
- Advanced molecular biology applications in fermentation.
Hybridoma Technology:
- Enabled production of monoclonal antibodies.
Process Simulations:
- Use of algorithms for optimizing industrial biotechnology processes.
Batch Fermentor
Batch Fermentor, a type of bioreactor used for growing microorganisms or cells under controlled conditions. Let's go through each part and its function within the system:
Key Components and Their Functions:
Motor:
- The motor drives the impeller (stirrer) inside the fermentor. It provides the necessary mechanical energy to keep the contents of the fermentor well-mixed, ensuring uniform distribution of nutrients, oxygen, and temperature throughout the liquid medium.
Impeller:
- The impeller is a rotating blade or series of blades that stir the liquid inside the fermentor. This mixing action helps to maintain homogeneity, preventing the formation of gradients (variations in temperature, pH, or nutrient concentration) within the reactor.
Cooling Jacket:
- The cooling jacket surrounds the fermentor vessel. It allows cooling water to flow through it, helping to regulate the temperature inside the fermentor. Temperature control is crucial for maintaining optimal conditions for the growth of microorganisms or cells.
Cooling Water Inlet and Outlet:
- Inlet: The cooling water inlet is where cold water enters the cooling jacket.
- Outlet: The cooling water outlet is where the warmed water exits after circulating through the jacket, carrying away excess heat from the fermentor.
Exhaust Air Filter:
- The exhaust air filter is placed on the outlet where air leaves the fermentor. It prevents contamination from entering the fermentor by filtering out any particles or microorganisms in the exhaust air.
Inlet Air Filter:
- The inlet air filter ensures that the air entering the fermentor is sterile, preventing contamination. This air is essential for supplying oxygen to the microorganisms or cells during aerobic fermentation.
Sparger:
- The sparger is located at the bottom of the fermentor and is responsible for introducing air (or oxygen) into the liquid medium. The sparger breaks the incoming air into fine bubbles, which helps in efficient oxygen transfer to the cells or microorganisms in the fermentor.
Baffles:
- Baffles are vertical plates attached to the inner wall of the fermentor. They disrupt the flow pattern created by the impeller, enhancing mixing and preventing the formation of a vortex. Baffles ensure that the liquid remains well-mixed, improving mass and heat transfer.
Rota Meter:
- The rota meter measures the flow rate of the air being supplied to the fermentor. It is a flowmeter that helps in maintaining the correct rate of air supply, which is crucial for providing adequate oxygen to the microorganisms or cells.
Process Overview:
In a batch fermentor, the growth medium and microorganisms (or cells) are added at the start of the process. The impeller, driven by the motor, continuously stirs the medium to maintain even distribution of nutrients and oxygen. The cooling jacket, along with the cooling water inlet and outlet, regulates the temperature, ensuring that the environment remains optimal for cell growth. The sparger introduces air into the medium, providing necessary oxygen for aerobic processes, while the inlet air filter ensures that the incoming air is sterile, preventing contamination.
Throughout the process, the exhaust air filter keeps the system sterile by filtering outgoing air. The baffles enhance mixing by preventing the formation of vortices. Finally, the rota meter monitors the flow rate of the incoming air, allowing for precise control of the oxygen supply.
This controlled environment enables the efficient growth of microorganisms or cells, leading to the production of desired metabolites or biomass, which can then be harvested at the end of the batch process.
Overview
A Batch Fermentor is a type of bioreactor where the entire process of fermentation or cell cultivation occurs in a single, closed system. The process begins by loading the fermentor with a precise amount of nutrients, inoculum (microorganisms or cells), and any other necessary components. Once everything is loaded, the fermentor is sealed, and the conditions inside (like temperature, pH, and oxygen levels) are tightly controlled throughout the process.
Key Features
- Closed System: No additional materials are added, nor is anything removed during the process. The only thing that changes is the internal composition of the medium as the microorganisms grow, consume nutrients, and produce products.
- Time-Limited Process: The process has a defined start and end. The batch is completed once the growth phase reaches a certain point or the desired product concentration is achieved.
- Productivity: The productivity in a batch system is typically measured by the amount of product produced at the end of the batch relative to the time it took. While it is simple to operate, it may not always be the most efficient in terms of productivity.
Advantages
- Simplicity: It’s straightforward to set up and operate, making it ideal for small-scale production or initial trials.
- Flexibility: Suitable for a wide range of different processes because each batch can be operated under different conditions.
- Contamination Control: The closed system minimizes the risk of contamination, which is critical when working with sensitive microorganisms.
Disadvantages
- Non-Continuous Operation: After each batch, the system must be cleaned, sterilized, and prepared for the next run, leading to downtime.
- Variable Quality: Since each batch is independent, there can be variability in product quality between batches.
Types of Bioreactors
1. Stirred Tank Bioreactor (STB):
Stirred Tank Bioreactor (STB), which is a commonly used bioreactor in industrial processes, especially in the fields of biotechnology and pharmaceuticals. Let’s go through the parts of the STB and their functions, as well as the overall process:
Key Components and Their Functions:
Motor:
- The motor drives the impeller, which is essential for mixing the contents inside the bioreactor. This mixing ensures that nutrients, oxygen, and other critical components are evenly distributed throughout the medium.
Impeller:
- The impeller is a mechanical agitator that stirs the contents of the bioreactor. It creates a homogeneous environment by keeping the cells and nutrients in suspension, which is crucial for efficient mass and heat transfer.
Sparger:
- The sparger introduces compressed air (or oxygen) into the bioreactor in the form of fine bubbles. These bubbles provide the necessary oxygen for aerobic microorganisms or cells to grow.
Cooling Jacket:
- The cooling jacket surrounds the vessel and is used to regulate the temperature within the bioreactor. Cold water flows through the jacket, absorbing excess heat generated by the metabolic activity of the microorganisms or by external heating sources like steam.
Cold-Water Inlet and Outlet:
- Inlet: This is where cold water enters the cooling jacket.
- Outlet: This is where the warmed water exits after circulating through the jacket, carrying away excess heat.
Temperature Probe:
- The temperature probe monitors the temperature inside the bioreactor. It provides real-time data that helps in maintaining the optimal temperature for the biological process.
pH Probe:
- The pH probe continuously measures the pH level of the culture medium. Maintaining the correct pH is crucial for the optimal growth of microorganisms, as pH affects enzyme activity and overall metabolic rates.
Oxygen Concentration Probe:
- This probe measures the concentration of dissolved oxygen in the medium. Adequate oxygen levels are essential for aerobic processes, and this probe ensures that the oxygen supply meets the demand of the growing culture.
Pressure Gauge:
- The pressure gauge monitors the internal pressure within the bioreactor. Controlling pressure is important for maintaining the integrity of the vessel and ensuring that the process conditions remain stable.
Acid/Base Addition:
- Acid or base can be added automatically to control the pH within the bioreactor. This system is often linked to the pH probe, which triggers the addition of acid or base to maintain the desired pH level.
Nutrient or Inoculant Addition:
- Nutrients or inoculants (starter cultures of microorganisms or cells) are introduced into the bioreactor to initiate and sustain the biological process. This can be done at the start or during the process in fed-batch or continuous modes.
Antifoam Addition:
- Foam can be a problem in bioreactors, as it can interfere with gas exchange and lead to loss of culture medium. Antifoam agents are added to prevent or reduce foam formation.
Filtered Waste Gases:
- As the bioreactor operates, gases are produced and need to be vented. The exhaust gases are filtered to remove any particulates or microorganisms before being released, preventing contamination and ensuring environmental safety.
Harvest Pipe:
- The harvest pipe is used to remove the culture medium or the final product from the bioreactor once the process is complete. This allows for the easy collection of the desired biological product.
Steam Inlet:
- Steam is used for sterilization before the process begins. It ensures that the bioreactor is free from any unwanted microorganisms that could contaminate the culture.
Process Overview:
In a Stirred Tank Bioreactor (STB), the process begins with the sterilization of the vessel using steam. Once sterilized, sterile nutrient medium and the inoculant (microorganisms or cells) are added. The motor drives the impeller to continuously stir the contents, ensuring that the cells remain suspended and that the nutrients and oxygen are evenly distributed.
Compressed air is introduced through the sparger, supplying the necessary oxygen for the cells. The oxygen concentration probe monitors the oxygen levels, while the pH probe and temperature probe keep track of the pH and temperature, respectively. These probes provide data to control systems that adjust the addition of acid/base, nutrients, or antifoam as needed.
The cooling jacket, regulated by the cold-water inlet and outlet, maintains the optimal temperature for the biological process. Pressure is monitored to ensure the vessel remains within safe operating limits.
Throughout the process, waste gases are filtered and vented safely. Once the desired product is formed, the culture is harvested through the harvest pipe.
This carefully controlled environment allows for the efficient production of biological products such as enzymes, pharmaceuticals, or biofuels.
Key Features
- Mixing: The primary function of STB is to provide efficient mixing. This ensures that all cells are exposed to the same environment, preventing any gradients (variations in temperature, pH, oxygen, etc.) from forming.
- Aeration: In aerobic processes, oxygen needs to be supplied continuously. STBs often have spargers (devices that release gas) at the bottom to introduce oxygen, which is then distributed by the stirring action.
- Control: STBs allow for precise control over various parameters, including temperature, pH, oxygen concentration, and nutrient levels. This control is crucial for optimizing the growth and productivity of the culture.
Operation Modes
- Batch Mode: In batch mode, the operation is similar to a batch fermentor, where everything is loaded at the start, and no additional inputs are added during the process.
- Fed-Batch Mode: In fed-batch mode, nutrients or substrates are added gradually during the process without removing any culture medium. This allows for extended culture times and higher product yields.
- Continuous Mode: In continuous mode, fresh medium is continuously added while the culture broth is simultaneously removed. This allows for a steady state where the growth rate and product formation can be maintained at an optimal level for extended periods.
Advantages
- Scalability: STBs can be easily scaled up for industrial production, from a few liters to several thousand liters.
- Flexibility: They can be used for a wide range of biological processes, including the production of enzymes, pharmaceuticals, and biofuels.
- Efficient Mixing: The stirring mechanism ensures that the culture is homogeneous, which is critical for consistent product quality.
Disadvantages
- Shear Stress: The mechanical action of stirring can create shear forces that may damage delicate cells, such as mammalian cells.
- Energy Consumption: The constant stirring and aeration require significant energy, making these systems more costly to operate compared to other bioreactor designs.
Application in Industry
- Biopharmaceuticals: Batch fermentors and STBs are widely used in the production of biopharmaceuticals, where the ability to control and optimize the growth environment is critical.
- Food and Beverage Industry: These bioreactors are also used in the production of fermented products, such as yogurt, beer, and wine.
- Biofuels: The production of biofuels, such as ethanol or biodiesel, often uses STBs for the large-scale cultivation of microorganisms.
2. Airlift Reactors
 |
| AIRLIFT BIOREACTOR |
Airlift Bioreactor with an external recirculation loop. This type of bioreactor is commonly used in various industrial applications for culturing cells or microorganisms, especially when gentle mixing is required.
Key Components and Their Functions:
Inlet Air:
- Air is introduced into the bioreactor through the inlet at the bottom. This air is typically supplied by a sparger or nozzle, which disperses the air into fine bubbles that rise through the liquid medium.
Nozzle:
- The nozzle helps direct the air into the bioreactor in a controlled manner, ensuring efficient mixing and distribution of oxygen within the medium.
Recirculation Loop:
- The recirculation loop is an external pathway that allows the liquid medium to be circulated back into the bioreactor. This loop helps enhance the circulation within the reactor, improving mixing and oxygen transfer.
Pump:
- The pump drives the recirculation loop, ensuring that the liquid is continuously cycled through the reactor. This helps maintain a uniform environment within the bioreactor, which is crucial for the growth and productivity of the cells or microorganisms.
Effluent Gas:
- The gas that rises through the liquid medium and exits the bioreactor is known as effluent gas. This gas may contain excess oxygen and byproducts such as carbon dioxide. It is often vented out and may be treated before release.
Process Overview:
In an airlift bioreactor, the air introduced at the bottom causes the liquid medium to circulate within the reactor. The rising air bubbles create a lower-density region in the center of the reactor, known as the riser, where the liquid flows upward. The denser, gas-free liquid in the outer region, known as the downcomer, flows downward. This natural circulation helps mix the contents of the bioreactor without the need for mechanical stirring, which reduces shear stress on the cells.
The recirculation loop, driven by the pump, enhances this circulation, ensuring that the entire volume of liquid is thoroughly mixed and oxygenated. This design is particularly useful for large-scale operations, where uniform conditions are essential for consistent and efficient production.
Airlift bioreactors are favored in processes where low shear stress and efficient oxygen transfer are critical, such as in the cultivation of shear-sensitive cells or when producing high-value biological products.
- Applications: Airlift reactors are ideal for processes requiring high oxygen transfer rates, such as aerobic cultures. They are also suitable for both free and immobilized cells.
- Advantages: These reactors have lower energy requirements compared to mechanically stirred reactors and are less prone to shear stress, making them suitable for shear-sensitive cells.
3. Packed Bed Reactors:
Packed Bed Reactor, which is a type of bioreactor commonly used in chemical processes, including bioprocessing, where reactions occur on the surface of solid catalysts. Here’s a detailed explanation of the process and the parts of the packed bed reactor:
Key Components and Their Functions:
Reactants Inlet:
- The reactants (which could be gases or liquids) enter the reactor from the bottom. These reactants are the substances that will undergo a chemical or biochemical reaction within the reactor.
Diffuser:
- The diffuser at the bottom of the reactor ensures that the reactants are evenly distributed as they enter the packed bed. It helps in achieving a uniform flow of reactants through the catalyst bed, which is essential for efficient reactions.
Catalyst on Support:
- The main section of the packed bed reactor contains a bed of solid particles, typically catalytic materials supported on a substrate. The catalyst provides a surface where the reactants can adsorb and react. This packed bed maximizes the surface area available for the reaction to occur.
- The catalyst on support is usually a porous material that allows reactants to pass through while providing a large surface area for the reaction. Common materials include activated carbon, silica, or alumina, often impregnated with the active catalyst material (e.g., metals like platinum, palladium, or enzymes in bioreactors).
Products and Unreacted Materials Outlet:
- As the reactants pass through the packed bed, they undergo chemical or biochemical reactions on the catalyst surface. The products of these reactions, along with any unreacted materials, exit the reactor from the top. These products are then sent for further separation and processing.
Process Overview:
In a packed bed reactor, the reactants are introduced at the bottom and flow upwards (or sometimes downwards) through the bed of solid catalyst particles. As they move through the bed, the reactants interact with the catalyst surface, where the chemical or biochemical reaction occurs.
- Reaction: The catalyst provides active sites that facilitate the conversion of reactants into products. The design ensures that the reactants have maximum contact with the catalyst surface as they flow through the reactor.
- Flow: The uniform flow of reactants, ensured by the diffuser, is crucial for maintaining consistent reaction rates throughout the packed bed. If the flow is uneven, some regions of the bed might be underutilized, leading to inefficient reactions.
- Product Formation: As the reaction progresses, the products form and are carried along with any unreacted materials towards the top of the reactor. The mixture of products and unreacted reactants is then directed to a separation unit, where they are separated and purified as needed.
Applications:
Packed bed reactors are widely used in various industrial processes, including:
- Chemical Synthesis: For processes like hydrogenation, oxidation, and other catalytic reactions.
- Bioprocessing: In processes like immobilized enzyme reactors where enzymes are fixed on a solid support within the packed bed.
- Waste Treatment: In bioreactors used for treating wastewater or gaseous pollutants, where the contaminants react with the solid catalysts.
Advantages:
- High Surface Area: The packed bed structure provides a large surface area for reactions, leading to high conversion rates.
- Efficient Use of Catalyst: The solid catalyst is stationary and remains within the reactor, allowing for continuous operation over long periods.
- Simple Design: The reactor design is relatively straightforward and scalable, making it suitable for a wide range of applications.
Challenges:
- Pressure Drop: The packed bed structure can cause a significant pressure drop across the reactor, which may require higher energy input to maintain the flow of reactants.
- Channeling: If the packing is not uniform or the flow is not well-distributed, the reactants may bypass parts of the bed, leading to inefficient use of the catalyst.
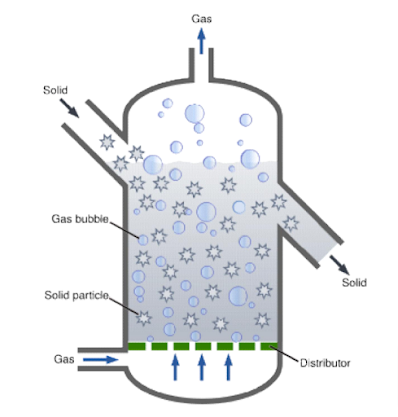
4. Fluidized Bed Reactors (FBB):
Fluidized Bed Bioreactor (FBB), which is a type of bioreactor commonly used in various chemical and biochemical processes, particularly for reactions involving solid catalysts or particles. Let’s break down the parts and process of a fluidized bed bioreactor:
Key Components and Their Functions:
Gas Inlet:
- The gas inlet at the bottom of the reactor introduces a gas (often air or another reactant gas) into the bioreactor. The gas flows upward through the reactor and plays a crucial role in the fluidization process.
Distributor:
- The distributor is a perforated plate or a mesh located at the bottom of the reactor. Its primary function is to evenly distribute the incoming gas across the cross-section of the reactor. This ensures that the gas flow is uniform, which is essential for proper fluidization of the solid particles.
Solid Particles:
- The solid particles in the fluidized bed typically consist of catalyst particles, biomass, or other materials that need to be suspended in the reactor. In biological processes, these could be immobilized enzymes or cells attached to a solid carrier.
Gas Bubbles:
- As the gas passes through the distributor, it forms bubbles that rise through the bed of solid particles. These gas bubbles create a fluidized state by lifting the solid particles, causing them to behave like a fluid. This fluidization increases the surface area available for reactions between the gas and the solid particles.
Solid Outlet:
- The solid outlet allows for the removal of spent or processed solid particles from the reactor. This can be used to control the residence time of solids in the reactor or to remove materials that have undergone a reaction.
Gas Outlet:
- The gas outlet at the top of the reactor allows the effluent gas to exit the system. This gas may carry reaction products, unreacted gases, and any volatilized materials from the reactor. It is often passed through filters or scrubbers before being released or recirculated.
Process Overview:
In a fluidized bed bioreactor, the process begins when gas is introduced through the inlet at the bottom. As the gas passes through the distributor, it is evenly spread across the reactor, forming bubbles as it rises through the bed of solid particles.
Fluidization: The gas bubbles lift and suspend the solid particles, creating a fluid-like state. This fluidization ensures that the solid particles are well-mixed and that the gas comes into intimate contact with the particles, promoting efficient mass and heat transfer.
Reaction: As the gas and solid particles interact, chemical or biochemical reactions occur. The fluidized state enhances these reactions by increasing the contact surface area and ensuring uniform temperature and concentration gradients within the reactor.
Product Formation: The reactions produce desired products, which may remain with the solid particles or be carried away by the gas stream. The solid particles can be collected and removed through the solid outlet, while the gas, containing any gaseous products or unreacted reactants, exits through the gas outlet.
Applications:
Fluidized bed bioreactors are widely used in:
- Chemical Processing: For catalytic reactions such as hydrocarbon cracking, polymerization, or Fischer-Tropsch synthesis.
- Biotechnology: In processes like aerobic fermentation, wastewater treatment, and bioremediation, where biomass needs to be kept suspended and well-oxygenated.
- Environmental Engineering: In processes like gasification and pyrolysis, where solid materials are converted into gases or liquids.
Advantages:
- High Surface Area: The fluidization process increases the contact area between the gas and solid particles, leading to efficient reaction rates.
- Uniform Conditions: The well-mixed nature of fluidized beds ensures uniform temperature and concentration throughout the reactor, minimizing hotspots and dead zones.
- Scalability: Fluidized bed reactors can be scaled up for industrial applications, handling large volumes of solids and gases efficiently.
Challenges:
- Complex Flow Dynamics: The fluidized state can be difficult to control, especially in terms of maintaining uniform fluidization without causing particle entrainment or channeling.
- Erosion of Particles: The continuous movement of particles can lead to attrition and erosion, which may affect the performance of the catalyst or other solid materials over time.
Comparison and Selection of Bioreactors
When selecting a bioreactor for industrial applications, several factors must be considered:
- Type of Biological Process: Different bioreactors are suited for different processes. For example, STBs are versatile and can be used for a variety of processes, while FBBs are preferred when high mass transfer is required.
- Shear Sensitivity: Some cells, like animal and plant cells, are shear-sensitive and may require reactors with lower shear stress, such as airlift or bubble column reactors.
- Oxygen Transfer: For aerobic processes, reactors like airlift and bubble column reactors, which provide high oxygen transfer rates, are preferred.
- Scalability and Cost: STBs are easy to scale up, making them ideal for large-scale production. However, their higher energy consumption and shear stress may be drawbacks in certain applications.
Conclusion
Each bioreactor type has its strengths and weaknesses, and the choice of reactor depends on the specific requirements of the biological process. Understanding these differences is crucial for optimizing production and achieving the desired outcomes in industrial applications.
Modes of Operation of Bioreactors
Bioreactors can operate in several different modes, each with specific characteristics and applications. The main modes of operation include:
- Batch Process
- Fed-Batch Process
- Continuous Process
- Continuous with Recycle
1. Batch Process
Overview:
- Closed System: In a batch process, the bioreactor is a closed system where a fixed amount of sterilized nutrient medium is introduced at the beginning. There is no further addition or removal of substrate during the process.
- Inoculation and Incubation: The medium is inoculated with microorganisms, and the fermentation process is allowed to proceed for a specific period. The conditions (like temperature, pH, oxygen) are controlled to optimize the growth and metabolic activities of the microorganisms.
- Phases of Growth: Throughout the fermentation, the microorganisms pass through different phases of growth, affecting the composition of the culture medium. The cells grow, consume nutrients, and produce biomass and metabolites.
- Completion and Harvesting: The process continues until the nutrients are exhausted or a predetermined time is reached. The culture broth is then harvested, and the desired product is extracted.
Key Characteristics:
- Constant Liquid Volume: The liquid volume in a batch reactor remains constant throughout the process.
- No Input/Output During Process: There is neither input nor output of the liquid after the process begins, except for necessary additions like oxygen or antifoaming agents.
- Operating Costs: The cost of running a batch reactor is influenced by the time required to reach the desired product concentration. Faster reactions reduce operating costs.
Applications and Advantages:
- Flexibility: Batch processes are versatile and can be adapted to various types of products and processes.
- Simplicity: The simplicity of operation makes batch reactors suitable for small-scale production and experimental setups.
Challenges:
- Limited Productivity: Since the process is stopped after each batch, the productivity is lower compared to continuous processes.
- Variability: There can be batch-to-batch variability, affecting the consistency of the product.
2. Fed-Batch Process
Overview:
- Intermediate System: The fed-batch process is a hybrid between batch and continuous processes. Nutrients are added to the bioreactor during the fermentation process, but there is no removal of the culture broth.
- Controlled Addition: The addition of nutrients is carefully controlled to avoid inhibitory effects and to extend the growth phase of the microorganisms.
- Extended Cultivation: By adding fresh nutrients, the microorganisms continue to grow and produce the desired product for a longer time than in a simple batch process.
Key Characteristics:
- Variable Liquid Volume: The volume of the liquid in the reactor increases as nutrients are added during the process.
- Enhanced Productivity: Fed-batch processes often lead to higher product yields compared to batch processes.
Applications and Advantages:
- Productivity: It allows for higher cell densities and product concentrations, making it suitable for processes where the product is either a growth-associated metabolite or secondary metabolite.
- Control: Provides better control over the environment within the reactor, leading to more consistent product quality.
Challenges:
- Complex Operation: Requires more sophisticated monitoring and control systems to manage the timing and rate of nutrient addition.
- Risk of Contamination: Extended operation time increases the risk of contamination.
3. Continuous Process
Overview:
- Open System: In a continuous process, fresh nutrient medium is continuously added to the bioreactor, while an equivalent volume of culture broth is continuously removed.
- Steady State: The reactor operates at a steady state, meaning the concentration of nutrients, cells, and products remains constant over time.
- Continuous Stirred-Tank Reactor (CSTR): This type of reactor is well-mixed, ensuring uniform conditions throughout the reactor. The product stream has the same composition as the liquid inside the reactor.
Key Characteristics:
- Constant Volume: The volume of the liquid remains constant because the input and output rates are balanced.
- High Productivity: Continuous processes are highly productive as the reactor operates indefinitely without the need to stop and restart.
Applications and Advantages:
- Efficiency: Continuous reactors are efficient for large-scale production, where high volumes of product are needed consistently.
- Consistency: The steady-state operation leads to consistent product quality.
Challenges:
- Complex Control: Maintaining steady-state conditions requires precise control over the flow rates and environmental conditions.
- High Setup Costs: Continuous processes require more sophisticated equipment and control systems, increasing initial setup costs.
4. Continuous Process with Recycle
Overview:
- Enhanced Continuous Operation: In this mode, part of the culture broth is recycled back into the bioreactor. This recycling can help maintain high cell densities and improve the efficiency of nutrient utilization.
- Applications: Commonly used in wastewater treatment and other processes where high cell densities are desirable.
Key Characteristics:
- Recycling: Recycling increases the effective biomass concentration in the reactor, improving the overall process efficiency.
- Steady-State Enhancement: The steady-state conditions can be maintained or even enhanced with the help of recycling, leading to better process control and productivity.
Applications and Advantages:
- Improved Nutrient Utilization: Recycle systems make better use of available nutrients, leading to more efficient processes.
- High Cell Density: Suitable for processes that benefit from high biomass concentrations, such as biofuel production or wastewater treatment.
Challenges:
- Complexity: The addition of a recycle loop adds complexity to the process, requiring more advanced monitoring and control systems.
- Potential for Contamination: Recycling introduces the potential for contamination to be reintroduced into the reactor.
Specific Continuous Operation Techniques
- Chemostat:
- In a chemostat, the liquid volume is kept constant by setting the inlet and outlet flow rates equal. The dilution rate is constant, and a steady state is achieved where the concentration of cells and nutrients is stable.
- Turbidostat:
- In a turbidostat, the biomass concentration is kept constant by adjusting the flow rate. Unlike a chemostat, the dilution rate in a turbidostat is variable, depending on the biomass concentration.
Conclusion
The choice of bioreactor operation mode depends on the specific needs of the production process. Batch processes are simple and flexible, fed-batch processes enhance productivity by extending the growth phase, and continuous processes offer high productivity and consistency for large-scale production. Continuous processes with recycle loops add further efficiency and are used in specialized applications. Understanding these modes helps in selecting the appropriate system for the desired biotechnological application.
Instrumentation in Bioreactors
Bioreactors require precise control over various parameters to ensure that the biological processes, such as fermentation or cell culture, proceed efficiently. Key instrumentation in bioreactors includes temperature control systems, pH control systems, foam control systems, and basic control schemes.
1. Temperature Control
Temperature is a critical parameter in bioreactor operations because it directly impacts the growth of microorganisms and the production of the desired product. If not properly managed, temperature fluctuations can lead to the degradation of heat-sensitive proteins and the denaturation of the product.
- Heat Sources: In a bioreactor, heat is generated through metabolic activities of microorganisms and the mechanical stirring of the medium.
- Heat Management: Proper heat management is vital to maintain optimal growth conditions. This is achieved using:
- Heating Jackets: Surround the bioreactor to either heat or cool the medium as needed. At a large scale, steam jackets are often used for heating.
- Temperature Sensors: Various sensors like thermocouples, thermistors, and resistance thermometers are used to monitor temperature:
- Thermocouples: Inexpensive and provide rapid temperature readings but have lower accuracy and require regular calibration.
- Thermistors: Semiconductor devices that are very sensitive to temperature changes and are relatively inexpensive.
- Platinum Resistance Thermometers: Preferred for high precision and stability, usually encased in stainless steel to avoid contamination.
2. pH Control
pH control is another crucial aspect of bioreactor operation. Microorganisms typically require a specific pH range to thrive, and deviations from this range can negatively affect enzyme activity and overall microbial health.
- pH Changes: pH can fluctuate due to:
- Overfeeding Substrate: Leads to the production of organic acids, lowering the pH.
- Carbohydrate Deficiency: Causes cells to metabolize proteins, leading to the production of ammonia, which increases pH.
- Ammonia Consumption: During protein production, ammonia consumption can release protons, decreasing pH.
- pH Measurement:
- pH Electrodes: These are typically glass electrodes that contain a buffer solution. The electrode measures the electrical potential difference, which corresponds to the pH level.
- pH Meters: Measure the difference in electrical potential between the pH electrode and a reference electrode, providing a potentiometric reading of the solution’s acidity.
3. Foam Control
Foam formation during fermentation is undesirable because it can lead to the loss of the fermentation broth and hinder accurate measurements and analyses. To manage foam:
- Antifoam Agents: These are added based on sensor feedback to prevent excessive foam formation. However, overdosing must be avoided as it can negatively affect mass transfer and overall process efficiency.
- Mechanical Foam Breakers: These are devices like disk-type mixers installed at the top of the bioreactor to physically break down foam. They are particularly useful when foam formation is too intense for chemical agents alone to manage.
4. Basic Control Schemes
Control systems in bioreactors ensure that processes run smoothly and conditions remain within desired ranges. There are several control strategies:
Open-Loop Control (Feedforward):
Feedforward Control, also known as Open-Loop Control. This type of control system is commonly used in various industrial and engineering applications where a predetermined control action is applied without considering feedback from the output.
Explanation of Feedforward Control (Open-Loop Control):
1. Basic Components:
Feedforward Controller:
- The feedforward controller is the system component that determines the control action to be applied to the process. It does so based on a model or understanding of the process dynamics and the anticipated disturbances.
Input:
- The input is the control signal generated by the feedforward controller. This input is applied directly to the process to achieve the desired output.
Process:
- The process is the system or operation being controlled. It could be anything from a chemical reaction to temperature control in a furnace, depending on the application.
Output:
- The output is the result or product of the process. In a feedforward control system, the output is not directly measured or used to adjust the input signal, which is why it's considered an open-loop system.
2. How Feedforward Control Works:
Prediction-Based Control:
- Feedforward control operates on the principle of prediction. The controller predicts the effect of disturbances on the process and takes preemptive action to counteract these effects before they impact the output.
No Feedback Mechanism:
- Unlike feedback control systems, where the output is continuously monitored and adjustments are made based on deviations from the desired setpoint, feedforward control does not rely on feedback from the output. The control action is determined entirely by the anticipated changes or disturbances.
Application in Processes:
- Feedforward control is particularly useful in processes where disturbances can be predicted accurately, and where immediate correction is required without waiting for feedback. For example, in a heating system, if the outside temperature is expected to drop, the feedforward controller can increase the heat supply in advance to maintain the indoor temperature.
3. Advantages of Feedforward Control:
- Fast Response:
- Because the controller acts in anticipation of disturbances, it can respond quickly to changes, often faster than a feedback system could.
- Stability:
- Since feedforward control does not depend on feedback, it avoids issues related to feedback loop stability, such as oscillations or delays.
4. Limitations of Feedforward Control:
- Accuracy Dependence:
- The effectiveness of feedforward control relies heavily on the accuracy of the process model and the ability to predict disturbances. If the model is inaccurate or the disturbances are unpredictable, the control action may not be effective.
- No Error Correction:
- Since there is no feedback, any errors in the process or deviations from the desired output cannot be corrected. This can be a significant drawback if the system experiences unexpected disturbances.
Closed-Loop Control (Feedback):
Feedback Control, also known as Closed-Loop Control. This type of control system is widely used in industrial processes, automation, and various engineering applications due to its ability to self-correct and maintain desired output levels by continuously monitoring the process.
Explanation of Feedback Control (Closed-Loop Control):
1. Basic Components:
Controller:
- The controller is the brain of the feedback control system. It receives the error signal (the difference between the desired setpoint and the actual measured output) and determines the appropriate action to minimize this error. The controller could be a simple proportional-integral-derivative (PID) controller, or a more complex system depending on the application.
Actuator:
- The actuator is the mechanism that the controller uses to influence the process. It could be a valve, a motor, a pump, or any device that can adjust the process variable to bring the output closer to the desired setpoint.
Process:
- The process is the system or operation being controlled. It could be anything from the temperature in a furnace to the speed of a motor, depending on the application. The process takes the control input from the actuator and produces an output.
Measured Output (Sensor):
- The sensor continuously monitors the output of the process and provides feedback to the controller. The measured output is crucial because it allows the system to determine if the desired setpoint is being achieved.
Error:
- The error is the difference between the desired setpoint and the measured output. The controller uses this error signal to adjust the actuator's input to the process, with the goal of reducing the error to zero (or as close as possible).
2. How Feedback Control Works:
Continuous Monitoring:
- In a feedback control system, the output of the process is continuously monitored by sensors. This output is compared to the desired setpoint (the target value the system is trying to maintain).
Error Calculation:
- The difference between the desired setpoint and the actual measured output is calculated, creating an error signal. If the actual output deviates from the setpoint, an error is generated.
Correction via Controller:
- The error signal is fed back into the controller. The controller analyzes this error and decides how to adjust the input to the process. The controller’s goal is to minimize the error by making the necessary adjustments to the process.
Actuator Response:
- The controller sends a command to the actuator, which makes the appropriate changes to the process (e.g., opening or closing a valve, increasing or decreasing speed). This adjustment is intended to bring the output back in line with the desired setpoint.
Self-Correction:
- The process output changes in response to the actuator’s adjustment. This new output is again measured by the sensor, creating a new feedback loop. The system continues to monitor, adjust, and correct itself in this manner, maintaining stability and the desired output.
3. Advantages of Feedback Control:
Automatic Error Correction:
- Feedback control systems automatically correct any deviations from the desired setpoint, ensuring the process remains stable and on target.
Robustness:
- These systems are robust and can handle disturbances and changes in the process conditions without requiring manual intervention.
Precision:
- Feedback control allows for precise control of process variables, making it ideal for applications where accuracy is critical.
4. Limitations of Feedback Control:
Potential for Oscillation:
- If not properly tuned, feedback control systems can oscillate, where the output continually overshoots and undershoots the setpoint.
Delayed Response:
- The system’s response can be slower than a feedforward system because it reacts to errors rather than anticipating them. This can be an issue in processes requiring rapid adjustments.
Combined Feedforward and Feedback Control:
- Concept: This approach integrates both feedforward and feedback controls, using model-supported control systems to predict and adjust for disturbances before they affect the process, while also correcting deviations in real-time.
- Application: Provides the highest level of control, particularly in complex and dynamic bioprocesses.
Example Diagram
a schematic diagram of a temperature control system for a bioreactor or a similar vessel. Here’s a detailed explanation of the components and how the system works:
Key Components:
Controller:
- The controller is the central unit that regulates the temperature inside the bioreactor. It receives input from the temperature sensor (T) and adjusts the flow of heating or cooling fluid accordingly to maintain the desired temperature.
Temperature Sensor (T):
- This sensor is placed inside the bioreactor and continuously measures the temperature of the liquid or medium within the reactor. The sensor sends this data to the controller.
Temperature Transmitter (TT):
- The temperature transmitter converts the signal from the temperature sensor into a standardized format that can be used by the controller. It ensures accurate and reliable temperature data is sent to the controller.
Heating and Cooling Valves (SRh and SRc):
- SRh (Heating Valve): This valve controls the flow of hot fluid (at 60°C) into the system. When the temperature inside the bioreactor is lower than the setpoint, the controller opens the SRh valve to allow hot fluid to flow, raising the temperature.
- SRc (Cooling Valve): This valve controls the flow of cold fluid (at 15°C) into the system. When the temperature inside the bioreactor is higher than the setpoint, the controller opens the SRc valve to allow cold fluid to flow, lowering the temperature.
Mixing Junction (I):
- The hot and cold fluids meet at a mixing junction (denoted as I) before entering the jacket or coils surrounding the bioreactor. This mixing allows precise temperature control, adjusting the temperature of the fluid that actually enters the bioreactor’s heating/cooling system.
Bioreactor:
- The vessel itself contains the medium or culture that requires temperature regulation. Inside the bioreactor, a stirrer is shown, which helps to maintain uniform conditions throughout the medium by mixing it continuously.
Inlet and Outlet for Fluids:
- The schematic shows that the mixed temperature-controlled fluid enters the bioreactor’s jacket or internal coils, while the outlet ensures that the heated or cooled medium is expelled after transferring its heat to the bioreactor contents.
Process Overview:
Temperature Measurement:
- The temperature sensor (T) inside the bioreactor constantly monitors the temperature of the medium. This data is sent to the temperature transmitter (TT), which forwards it to the controller.
Controller Action:
- The controller compares the measured temperature to the desired setpoint. If the temperature deviates from the setpoint, the controller takes action to correct it:
- If the temperature is too low: The controller opens the heating valve (SRh), allowing hot fluid (60°C) to flow into the system.
- If the temperature is too high: The controller opens the cooling valve (SRc), allowing cold fluid (15°C) to flow into the system.
- The controller compares the measured temperature to the desired setpoint. If the temperature deviates from the setpoint, the controller takes action to correct it:
Mixing and Temperature Regulation:
- The hot or cold fluids mix at the junction (I) to achieve the desired temperature before being circulated through the bioreactor’s jacket or coils. This fluid heats or cools the medium inside the bioreactor, adjusting its temperature as required.
Continuous Monitoring and Adjustment:
- The process is continuous, with the controller constantly receiving feedback from the temperature sensor and making real-time adjustments to maintain the desired temperature within the bioreactor.
example : pH Control System in a Bioreactor instead of temperature as shown in previous example.
Key Components:
Controller:
- The controller is the central unit that regulates the pH inside the bioreactor. It receives input from the pH sensor and adjusts the flow of acidic or basic solutions accordingly to maintain the desired pH level.
pH Sensor:
- The pH sensor is placed inside the bioreactor and continuously measures the pH of the liquid or medium within the reactor. This sensor sends real-time pH data to the controller.
pH Transmitter:
- The pH transmitter converts the signal from the pH sensor into a standardized format that the controller can interpret. This ensures accurate and reliable pH data is sent to the controller.
Acid and Base Valves (SRa and SRb):
- SRa (Acid Valve): This valve controls the flow of an acidic solution into the bioreactor. When the pH inside the bioreactor is higher than the setpoint (too basic), the controller opens the SRa valve to add acid, lowering the pH.
- SRb (Base Valve): This valve controls the flow of a basic solution into the bioreactor. When the pH inside the bioreactor is lower than the setpoint (too acidic), the controller opens the SRb valve to add a base, raising the pH.
Mixing Junction (I):
- Similar to the temperature control system, the acidic and basic solutions mix at a junction before being introduced into the bioreactor. This allows for precise pH control by adjusting the pH of the solution entering the bioreactor.
Bioreactor:
- The vessel contains the medium or culture that requires pH regulation. Inside the bioreactor, a stirrer ensures uniform conditions by continuously mixing the medium, helping to distribute the pH-adjusting solution evenly.
Process Overview:
pH Measurement:
- The pH sensor inside the bioreactor constantly monitors the pH of the medium. This data is sent to the pH transmitter, which forwards it to the controller.
Controller Action:
- The controller compares the measured pH to the desired setpoint. If the pH deviates from the setpoint, the controller takes corrective action:
- If the pH is too high (too basic): The controller opens the acid valve (SRa), allowing the acidic solution to flow into the bioreactor and lower the pH.
- If the pH is too low (too acidic): The controller opens the base valve (SRb), allowing the basic solution to flow into the bioreactor and raise the pH.
- The controller compares the measured pH to the desired setpoint. If the pH deviates from the setpoint, the controller takes corrective action:
Mixing and pH Regulation:
- The acid or base solution mixes at the junction (I) before being introduced into the bioreactor. This solution alters the pH of the medium, bringing it closer to the desired setpoint.
Continuous Monitoring and Adjustment:
- The process is continuous, with the controller constantly receiving feedback from the pH sensor and making real-time adjustments to maintain the desired pH within the bioreactor.
1. Criteria for a Good Medium
A good culture medium should:
- Yield a maximum amount of the desired product.
- Allow for a high rate of product formation.
- Minimize the formation of undesirable by-products.
- Be easy to prepare and sterilize.
- Pose minimal challenges during the fermentation process.
- Be consistently available and of high quality year-round.
2. Key Components of a Medium
The medium should include:
- Carbon Source: Provides energy and building blocks for product formation.
- Examples: Cane molasses, starch, glucose, and sucrose.
- Nitrogen Source: Supplies amino acids and energy.
- Examples: Urea, ammonium salts, soy bean meal, and corn steep liquor.
- Minerals: Essential for metabolic functions.
- Growth Factors: Needed for cell component synthesis.
- Water: Acts as the solvent.
3. Factors Influencing the Choice of Carbon Source
The choice of a carbon source depends on:
- Its metabolic rate and ease of utilization.
- The product type (e.g., ethanol, antibiotics).
- The cost and purity of the carbon source.
- Media preparation techniques.
- Regulatory or government constraints.
4. Sources of Carbon
- Saccharine Materials: Molasses, fruit juices, and cheese whey.
- Molasses (from sugarcane or beets) is rich in sucrose and nutrients but requires supplementation for deficient components.
- Starchy Materials: Cereals, roots, and tubers (e.g., potatoes, tapioca) require pretreatment to convert starch into fermentable sugars.
- Cellulosic Materials: Sulfite waste liquor and wood molasses provide hexoses and pentoses, but pretreatment is essential.
- Hydrocarbons and Vegetable Oils: Includes methane, methanol, and oils (e.g., soybean, maize oil) with high energy yield and volume advantages.
5. Sources of Nitrogen
Nitrogen sources are critical for growth and product formation:
- Inorganic Nitrogen Sources:
- Ammonia gas is used both for pH control and as a nitrogen source.
- Ammonium salts and nitrates provide acidic or alkaline drift during utilization.
- Organic Nitrogen Sources:
- Examples include corn steep liquor, soy bean meal, and distillers' solubles.
- They often contain complex nitrogen compounds and vitamins (e.g., B-complex).
6. Inducers and Inhibitors
- Inducers: Substances that activate the production of specific enzymes.
- Example: Starch induces amylase production; pectin induces pectinase.
- Inhibitors: When added, they may increase a specific product or accumulate intermediates.
- Example: Bromide increases tetracycline yield by inhibiting chlortetracycline production.
7. Precursors
- Chemicals added to the medium to enhance the desired product yield.
- Example: Corn-steep liquor increases penicillin yield by introducing phenylethylamine.
8. Antifoaming Agents
Foam is often problematic in fermentation processes due to:
- Protein-rich components in the medium.
- Biological activity during fermentation.
Issues with Foam:
- It can cause removal of cells and reduced mass/heat transfer.
- Leads to contamination risks and invalid sensor readings.
Antifoaming Agents:
- Include silicones, esters, and fatty acids that suppress foam formation and persistence.
Ideal Properties:
- Non-toxic, active at low concentrations, heat sterilizable, and cheap.
9. Other Additives
Additional components like buffers, chelators, and growth factors may be included based on the organism and product requirements.






Comments
Post a Comment