7.) The flounder (fish) is able to survive in very cold water, but only when water is in its liquid state. Discuss what properties of water in the solid state may prevent or harm life.
7.) The flounder (fish)) is able to survive in very cold water, but only when water is in its liquid state. Discuss what properties of water in the solid state may prevent or harm life.
A.)
However, the top surface of the water bodies freezes, and underneath it, the water remains as the liquid.
Generally, we know that all the liquids expand on the heating, but water bends the rule.
That is, when water is heated, instead of expanding the volume decreases gradually. This decrease in the volume of the water is seen up to the temperature rise
When the water is cooled down, it solidifies and the volume we observe the contraction in the volume as other liquids.
At the water has the maximum density. The ice starts to expand and the cooling continues till the temperature of , the point at which the ice starts to freeze out as a solid.
The water has an anomalous expansion property. This property of expansion creates a thick layer of ice above the surface of the lake or the pond. But the water beneath this thick layer of the ice does not freeze into a solid but remains as a liquid.
In other words, we can say that the water has the maximum density and least volume at the temperature. This is called the irregular expansion of the water and this resembles the anomalous expansion of water.
This expansion enables marine life (fishes or marine plants) which exist in the colder regions to survive, even when the water solidifies on the surface and below the surface is still liquid.
The general depiction of the layers of the ice in the pond in the colder region are as follows:
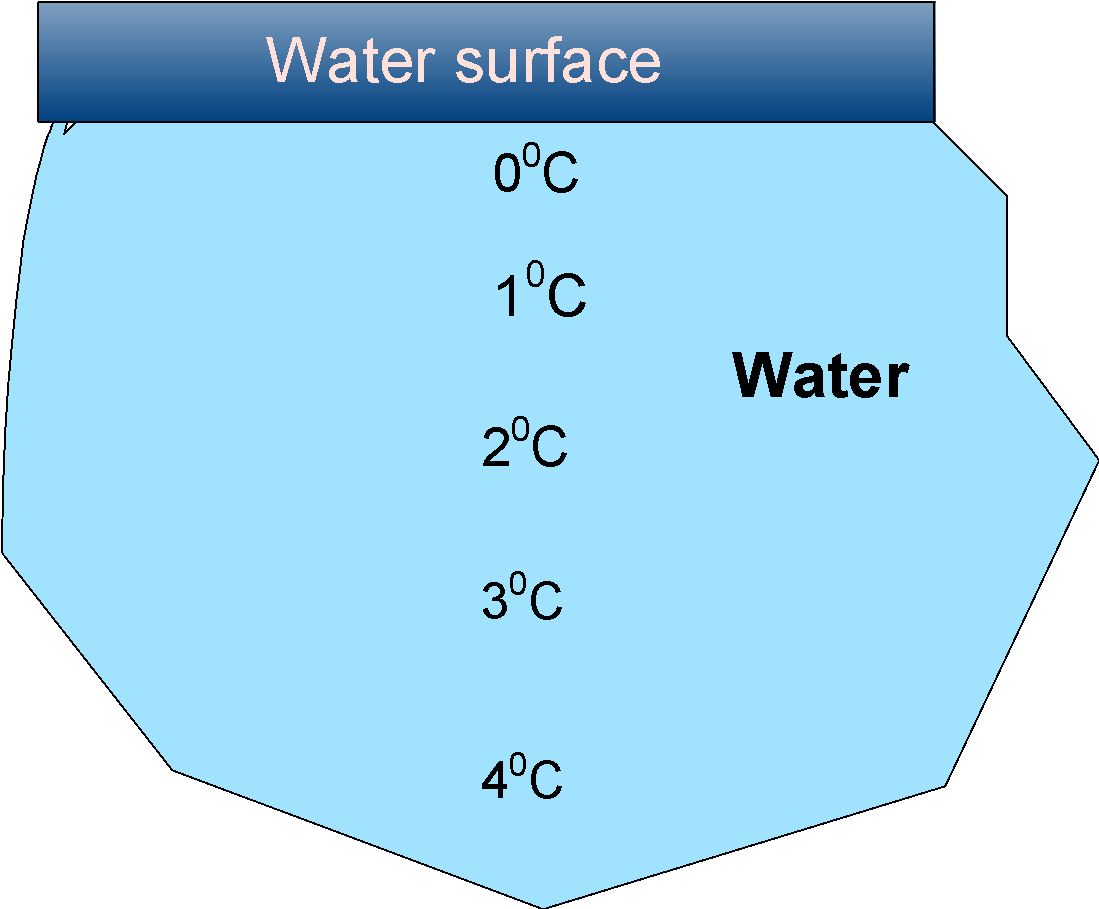
In the winter months, the temperature drops below the freezing point, and the upper layer starts to cool down. When the temperature reaches the water acquires a higher density, and then water sinks to the bottom. This displaces the water from the lower layer and these also cool down and sink again.
When the temperature goes below , the density of water decreases, and thus no more water sinks. Henceforth, the water at the top is at the and the bottom is .
These how the fish or the marine life is survived even under the thick layer of ice.
Note: Marine life has the dangers of freezing in the water. But, remember that the fish body can be solidified only when the temperature of the surrounding drops below the . Thus, the arctic, and Antarctic fishes can adjust to the surrounding interestingly. Some fishes may produce the antifreeze molecules known as the glycoprotein reduces the freezing point of body fluids.

Comments
Post a Comment